- Health at Bayer
-
Pharmaceuticals
- News & Stories
- Inspired By You
- Treatment Areas
- Innovation & Technologies
- Cell and Gene Therapy
-
Sustainability
- Patient Access Charter
- Leadership Perspective
- Strengthening Healthcare Access
-
Empowering Women, Globally
- Boosting Family Planning Usage through Digital Channels
- Capacity building: Addressing Root Causes through Partnerships
- Impact at Scale: The Challenge Initiative
- Promoting Awareness: World Contraception Day (WCD) & the Your Life Campaign
- Providing Accessible and Affordable Contraceptives
- Enabling Family Planning in Humanitarian Settings
- Fighting Neglected Tropical Diseases
- Moving Non-Communicable Diseases Care Forward
- Ensuring a Sustainable Product Supply
- Delivering Better Cancer Care
- Transparency
- Personal Health
- Report a Side Effect
- Medical Counterfeits
New Safety Features for Prescription Medicine in Europe
As of February 2019, a new European Medicines Verification System is in place with harmonized safety and control measures to prevent falsified medicines from reaching patients.
Technical implementation of the new process at Bayer’s production sites has already been completed and printing of the new verification code on prescription medicines is ongoing.

New Harmonized Safety and Control Measures
New safety features for prescription medicines will enable an end-to-end verification system to prevent falsified medicines from entering the legal supply chain. The new safety features will be mandatory in the EU Member States
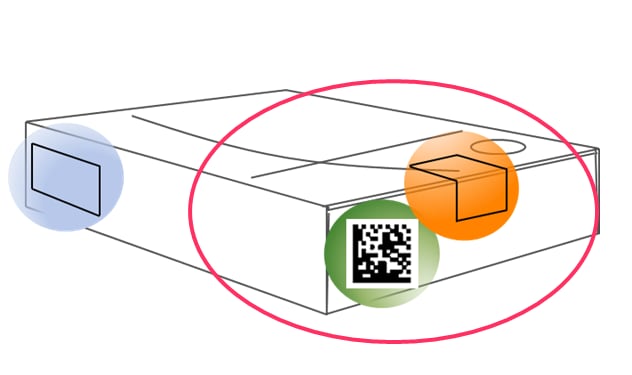
How to Recognize Genuine Products
Under the European Medicines Verification System, the authenticity of medicines will be guaranteed by a so-called Data Matrix Code that incorporates an unique identifier (UI) and an anti-tampering device.
At different stages in the distribution chain, e.g. when the medicine packages reach the pharmacies, the packages will be scanned, checked and verified for authenticity. If there is an alert, the package will not be supplied and there will be an investigation to determine whether the medicine has been falsified.
As the shelf life of each product varies, it will take 3-5 years until all packages have the new safety features. Therefore, you should not be worried if your current medicine does not have the new code.
Further information
Bayer´s Commitment to Fighting Counterfeit Drugs









