Development Pipeline

We are determined to drive breakthrough innovations for patients in diseases of high unmet medical need. In clinical development we are currently advancing more than 31 projects that we review regularly so that we can give priority to the most promising programs.
Pipeline Overview
The following table lists a selection of major Pharma portfolio projects that we are advancing through different stages of development. Click on a project for further information.
More detailed information on individual programs, therapeutic areas, and development timelines as well as a one-page overview of our development portfolio can be found in our overview package for download.
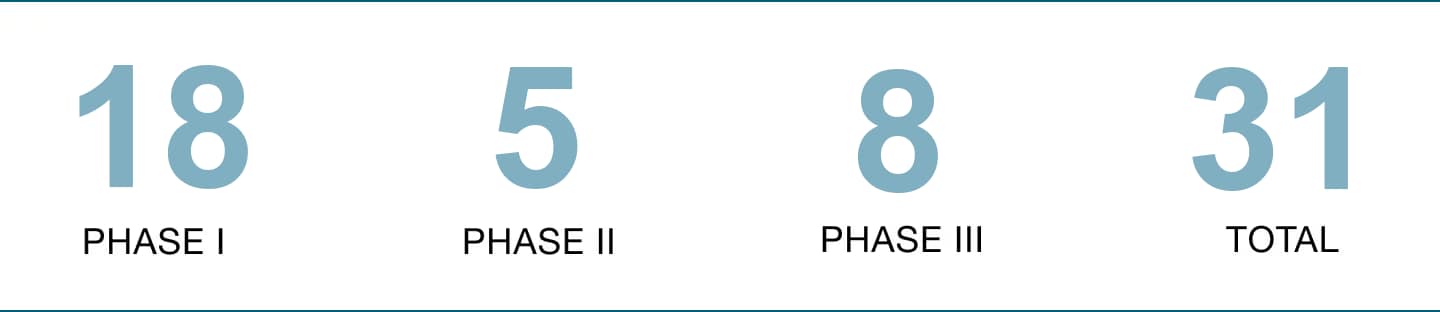
Number of programs in clinical development.
| Oncology (ONC) |
| Cardiovascular* (CVD) |
| Neurology & Rare Diseases (NRD) |
| Immunology (IM) |
| Others |
| Phase | Area | Program (Mode-of-action) | Indication | |
|---|---|---|---|---|
| III | ONC | Darolutamide (AR Inhibitor) | Adjuvant Prostate Cancer | |
| III | ONC | Darolutamide (AR Inhibitor) | Prostate Cancer with Biochemical Recurrence after Curative Radiotherapy | |
| III | ONC | Sevabertinib (HER2/mEGFR Inhibitor) | Advanced Non-small Cell Lung Cancer with HER2 Activating Mutations, 1L | |
| III | CVD | Finerenone (MR Antagonist) | Non-diabetic CKD (FIND-CKD) | |
| III | CVD | Finerenone (MR Antagonist) | Chronic Kidney Disease in Type 1 Diabetes (FINE-ONE) | |
| III | CVD | Vericiguat (sGC Stimulator) | Heart Failure (HFrEF) | |
| III | CVD | Asundexian (FXIa Inhibitor) | 2⁰ Stroke Prevention (OCEANIC Stroke) | |
| III | NRD | Bemdaneprocel (Cell Therapy) | Parkinson's Disease (exPDite-2) | |
| II | ONC | Sevabertinib (HER2/mEGFR Inhibitor) | Metastatic or Unresectable Solid Tumors with HER2-activating Mutations (PanSOHO) | |
| II | CVD | Congestive Heart Failure rAAV Gene Therapy (AB-1002) | Congestive Heart Failure (GenePHIT) | |
| II | CVD | Anti-a2AP | Acute Ischemic Stroke; Pulmonary Embolism (SIRIUS) | |
| II | CVD | Nurandociguat (sGC Activator Oral) | Chronic Kidney Disease (ALPINE-1) | |
| II | NRD | Parkinson's Disease rAAV Gene Therapy (AB-1005) | Parkinson's Disease (REGENERATE-PD) | |
| I | ONC | VVD Keap1 Act | Advanced Solid Tumors | |
| I | ONC | 225Ac-Pelgifatamab | Advanced Prostate Cancer | |
| I | ONC | 225Ac-PSMA-Trillium | Advanced Prostate Cancer | |
| I | ONC | SOS1 Inhibitor | Advanced Solid Cancers | |
| I | ONC | PRMT5 Inhibitor | MTAP-deleted Solid Tumors | |
| I | ONC | VVD RAS-PI3K Inhibitor | Advanced Solid Tumors | |
| I | ONC | 225Ac-GPC3 | Advanced Liver Cancer | |
| I | ONC | VVD WRN Inhibitor | Advanced Solid Tumors | |
| I | ONC | KRAS G12D Inhibitor | Advanced Solid Tumors | |
| I | CVD | SEMA 3a | Alport Syndrome | |
| I | CVD | Dual FIIa/Xa Inhibitor | Anti-coagulation | |
| I | CVD | GIRK4 Inhibitor | Atrial fibrillation | |
| I | NRD | Multiple System Atrophy rAAV Gene Therapy | Multiple System Atrophy | |
| I | NRD | Pompe Disease rAAV Gene Therapy | Pompe Disease | |
| I | NRD | LGMD2I/R9 rAAV Gene Therapy | Limb Girdle Muscular Dystrophy | |
| I | Other | GPR84 Antagonist | Diabetic Neuropathic Pain | |
| I | Other | BAY 2701250 | Pulmonary Hypertension | |
| I | Other | Primary Photoreceptor Diseases Cell Therapy | Primary Photoreceptor Disease |
* Including Precision Cardiovascular, Nephrology & Acute Care
![]()
![]() New molecular entity
New molecular entity![]() Life cycle management
Life cycle management





















